Is the EMA about to fully approve the vaxxines so that they can be mandated and everyone force-vaccinated?
A claim has been made that in Europe the pharmaceutical companies did not renew their request for a conditional marketing authorisation (CMA) and that therefore the Covid vaccines are no longer approved for use. According to a French article, they were supposed to do so six months in advance of the expiration of the one-year temporary conditional marketing authorisation. I have checked the cited EU regulations and this is correct (https://ec.europa.eu/health/sites/default/files/files/eudralex/vol-1/reg_2004_726/reg_2004_726_en.pdf):
6. In exceptional circumstances and on public health grounds the
Commission may grant exemptions from paragraphs 4 and 5. Such
exemptions must be duly justified.
7. Following consultation with the applicant, an authorisation may be
granted subject to certain specific obligations, to be reviewed annually
by the Agency. The list of these obligations shall be made publicly
accessible.
By way of derogation from paragraph 1, such authorisation shall be
valid for one year, on a renewable basis.
Commission may grant exemptions from paragraphs 4 and 5. Such
exemptions must be duly justified.
7. Following consultation with the applicant, an authorisation may be
granted subject to certain specific obligations, to be reviewed annually
by the Agency. The list of these obligations shall be made publicly
accessible.
By way of derogation from paragraph 1, such authorisation shall be
valid for one year, on a renewable basis.
COMMISSION REGULATION (EC) No 507/2006
of 29 March 2006 (https://ec.europa.eu/health/sites/default/files/files/eudralex/vol-1/reg_2006_507/reg_2006_507_en.pdf):
of 29 March 2006 (https://ec.europa.eu/health/sites/default/files/files/eudralex/vol-1/reg_2006_507/reg_2006_507_en.pdf):
(9) In accordance with Regulation (EC) No 726/2004, conditional marketing authorisations will be valid for one year
on a renewable basis. The deadline for submission of a
renewal application should be six months prior to the
expiry of the marketing authorisation, and the opinion of
the European Medicines Agency (hereinafter the Agency)
on the application should be adopted within 90 days of
its receipt. To ensure that medicinal products are not
removed from the market except for reasons related to
public health, the conditional marketing authorisation
should, as long as a renewal application is submitted
within the deadline, remain valid until the Commission
reaches a decision based on the renewal assessment
procedure.
on a renewable basis. The deadline for submission of a
renewal application should be six months prior to the
expiry of the marketing authorisation, and the opinion of
the European Medicines Agency (hereinafter the Agency)
on the application should be adopted within 90 days of
its receipt. To ensure that medicinal products are not
removed from the market except for reasons related to
public health, the conditional marketing authorisation
should, as long as a renewal application is submitted
within the deadline, remain valid until the Commission
reaches a decision based on the renewal assessment
procedure.
According to my data, the vaccines will not reach the end of their one-year conditional marketing authorisation period until this December, and the following January and March so I don’t see a justification for the claims made in both France and Italy (https://forlifeonearth.weebly.com/insights-into-the-european-public-data-on-cmas-30321.html):
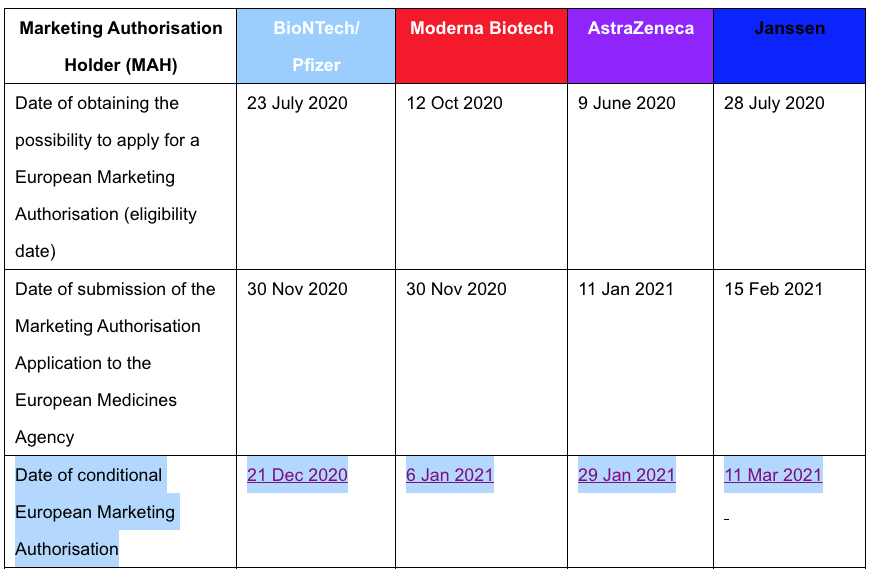
The article originating in Italy claims that the conditional marketing authorisation is no longer valid but the author does not provide the proof: In Italy The Vaxx Is No Longer Legally Approved For Use: https://www.fromrome.info/2021/08/16/in-italy-the-vaxx-is-no-longer-legally-approved-for-use/:
… That Conditional Approval for Commercial use authorization has expired, in law, with the official recognition by AIFA, the Italian Pharmaceutical Approval Agency, of the use of other monoclonial anti-bodies as an effective cure for SarsCov19 infection. …
In France, it is Dr. Philippe Chazournes who has said that the conditional marketing authorisation is no longer valid (https://qactus.fr/2021/08/20/urgent-le-docteur-philippe-de-chazourne-nous-lache-une-bombe-et-nous-explique-par-preuves-que-les-labos-nont-pas-renouvele-leur-autorisation/) and:

However, as stated above in EU regulation 507/2006
of 29 March 2006, “the conditional marketing authorisation
should, as long as a renewal application is submitted
within the deadline, remain valid”. So it seems to me that we have an anomalous situation here, in which, if the pharmaceutical companies have indeed failed to submit an application to renew, we do not know if the CMA remains valid or not. It would seem to depend on the choice of action of the European Medicines Agency (EMA). Britain, of course, after Brexit, is not part of this but nevertheless seems to follow suit with the actions of the European Union.
of 29 March 2006, “the conditional marketing authorisation
should, as long as a renewal application is submitted
within the deadline, remain valid”. So it seems to me that we have an anomalous situation here, in which, if the pharmaceutical companies have indeed failed to submit an application to renew, we do not know if the CMA remains valid or not. It would seem to depend on the choice of action of the European Medicines Agency (EMA). Britain, of course, after Brexit, is not part of this but nevertheless seems to follow suit with the actions of the European Union.
Given that we are in a situation of extreme uncertainty and deep suspicion of “authorities” by the general public after the abuse perpetrated on them in the last 18 months, there is, of course, another interpretation to be put on all of this. Could it be that the pharmaceutical companies effectively control the EMA and that they knew from the outset that the EMA would automatically grant them an unconditional marketing authorisation after a certain time? Despite the fact that 34,052 Covid-19 injection-related deaths and over 5.46 million injuries in EU/UK/US had been reported as at 1.8.2021 (https://blog.nomorefakenews.com/2021/08/17/massive-fraud-in-reporting-vaccine-injuries-withheld-data-pretense-of-safe/) and that it is universally acknowledged that the voluntary or “passive” reporting systems under-report vaccine adverse events by up to 100 times, such that the real number of deaths could be 3.5 million or more, they are going to give full approval for marketing authorisation shortly so that the vaccines can be mandated? This despite the fact that the deadlines for submitting confirmation of efficacy, safety and tolerability of the vaccines are between a year and three years away (https://forlifeonearth.weebly.com/insights-into-the-european-public-data-on-cmas-30321.html):

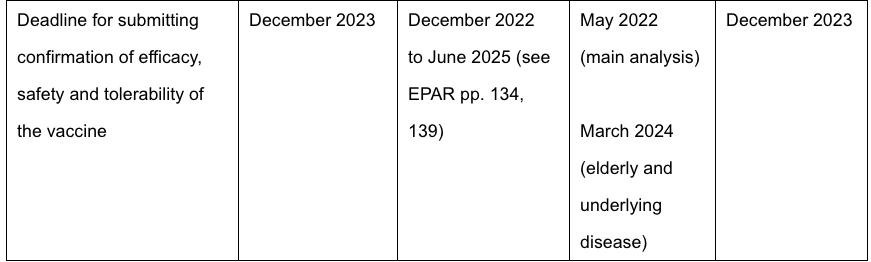
And despite the fact that the clinical trials are not due to be completed until between 2022 and 2025 (https://forlifeonearth.weebly.com/insights-into-the-european-public-data-on-cmas-30321.html).
I think we need more evidence of what is really going on to be provided. These claims from France and Italy urgently need validation.

New postings every day at https://forlifeonearth.weebly.com/
- The FLOE Show 1: Who’s pulling the strings on the Great Reset global putsch?
- ACTION
- Articles 5G & wireless technology
- 5G/COVID CONNECTION
- In historic, decision, federal court orders FCC to explain why it ignored scientific showing harm from wireless radiation, 13.8.21
- Former Vodafone boss blows whistle on 5G Coronavirus, 4.2.20
- 5G/WIRELESS TECHNOLOGY WEAPONS HYDRA
- COVIDIOCRACY
- Financial expert George Gammon explains how globalists plan to take your home, 17.8.21
- Putting Kids First – Helping Parents Address School Mandates
- Pass sanitaire – comment résister
- 7-minute cure for Covid misinformation (testimony of Dr. Dan Stock), 7.8.21
- Top 10 Pandemic Fables, 13.8.21
- EMPOWERMENT
- MUST-WATCH – scrubbed from the Internet: Knowledge NOT Sustainable! – UN Agenda 21/2030
- A Clarion Call to the Men of our World – It is Time to Restore the Sacred Masculine! 14.8.21
- The Psychology of Power – How to Dethrone Tyrants, 19.7.20
- GENOCIDE
- Most secret intelligence agency Deagel forecasts 70% depopulation of US by 2025 – to 100 mil.
- German doctors: kill shot & blood analysis under microscope, 17.8.21
- Dr. David Martin makes explosive claims of ‘patented genocide’
- Dr. Zev Zelenko slays globalists, exposes “global genocidal event” which will kill 75-90% of the vaxxed, 13.8.21
- MAINTAINING YOUR HEALTH IN A TIME OF CRISIS
- PUSHBACK USING THE LAW
- Andalusian court rules against Covid-free proof for entry to nightlife venues
- In historic, decision, federal court orders FCC to explain why it ignored scientific showing harm from wireless radiation, 13.8.21
- VAXX
- Dr. Lee Merritt joins the Health Ranger to discuss vaccine mind control & medical madness, 7.2021 (very interesting conversation!)
- Dopey Australian MP Victor Dominello thanks hospital staff for Bell’s Palsy, 17.8.21
- Stew Peters & Dr. Jane Ruby: German doctors examine blood & vials: horrific, 18.8.21
- Swiss university finds AstraZeneca vaxxine two thirds contaminated, June 2021
- Taliban have banned Covid vaccine in East Afghanistan’s Paktia – lucky Afghans! 13.8.21
- DARPA was secretly developing the mRNA vaccine years ago through Moderna, and they seek permanent control over your body and bloodline, 17.8.21
- Navy commander warns of national security threat from mandatory vaccination of US military, 19.8.21
- Doctor explains “shedding” or transmission from vaxxenated to unvaxxenated, 27.5.21
- Former Pfizer VP warns: Dr. Michael Yeadon discussed recent findings indicating that experimental Covid-19 vaccines concentrate in a woman’s ovaries and induce an ‘autoimmune attack’ on the placenta, 5.8.21
- 57 top scientists & doctors release shocking study on Covid vaccines & demand immediate stop to all vaccines, 17.8.21
- Australia: Max Igan: “Blockade everything!” 18.8.21
- TECHNO-TRANSHUMANISM
- WEF dystopian video – your prison future spiced up with enticing music
- The Pentagon is experimenting with AI that can predict events ‘days in advance’, 5.8.21
- Graphene Integrated Photonics (GIP) optical devices that aim to enable 5G to support rapidly increasing global data traffic volumes for 2024-2025
If you no longer wish to receive information about the Stop 5G campaign from this source,
just click “Reply” and say so. Thanks!
Si vous ne souhaitez plus recevoir d’informations de cette source sur la campagne Stop 5G,
cliquez simplement sur “Répondre” et me le dire. Merci!
Wenn Sie keine Informationen mehr über die Stop 5G Kampagne
von dieser Quelle erhalten möchten,
klicken Sie einfach auf “Antworten” und sagen es. Danke!